Search for information
Merger of ATA and DTA Reshapes the Landscape of Digital Healthcare ServicesMerger of ATA and DTA Reshapes the Landscape of Digital Healthcare Services
April 15, 2025, 2:53 pm EDT
Merger of ATA and DTA Reshapes the Landscape of Digital Healthcare Services

Source: Images from the Internet, if there is any infringement, please contact the removal of
Source: Images from the Internet, if there is any infringement, please contact the removal of

Source: Images from the Internet, if there is any infringement, please contact the removal of
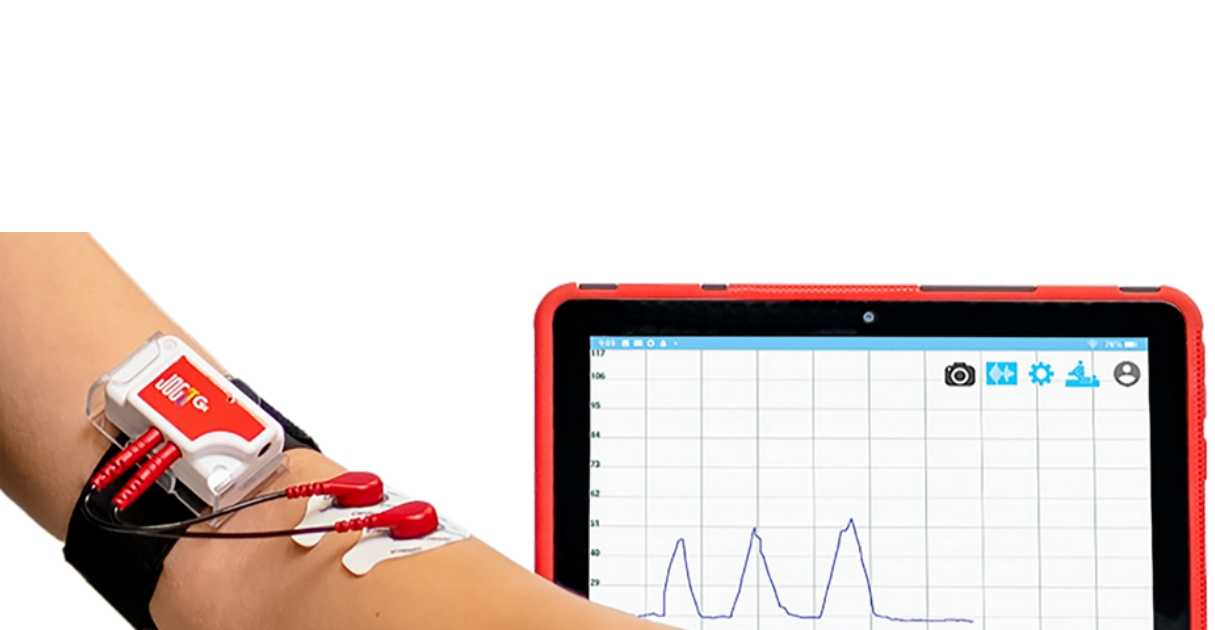
May 2nd: Visit the Digital Health Center of Stanford University, focusing on the R&D and design of AI digital health and wearable products, verification of the safety and effectiveness of DTx, and testing of AI algorithms.
May 2nd: Visit the NVIDIA Smart Hospital project and analyze the latest application cases of AI in medicine.
May 3rd - 6th: Participate in the 2025 NEXUS-ATA-DTx Digital Therapeutics and Telemedicine Annual Conference (Brief introductions of the enterprises for communication are as follows).
May 7th: The annual conference ends, and participants can make their own arrangements or return to China.
*The final itinerary is subject to the arrangements of the host party.

Source: Images from the Internet, if there is any infringement, please contact the removal of
02 Dario Health: An FDA-approved digital health solution for cardiometabolic conditions. It is a digital health platform (a listed company) offering cardiometabolic digital health solutions, including diabetes, hypertension, and weight management/weight loss in a virtual center model, with experience in a membership-based business model.
03 Cognito Therapeutics: An FDA-approved digital therapeutics company for the best DTx treatment of dementia. It develops the best digital therapeutics for early neurodegenerative diseases (dementia and Parkinson's disease) - AI-driven DTx and wearable devices.
04 Cognoa: An FDA-approved digital therapeutics solution for childhood autism. Its Canvas Dx is the first digital therapy for childhood autism and currently the only FDA-authorized digital auxiliary diagnosis solution, helping doctors diagnose or identify autism in children aged 1.5 to 6 years within a few days (instead of months or years).
05 MedRhythms: A leading global digital therapeutics company. It uses smart sensors, music, and digital software apps to build evidence-based neurofeedback interventions, aiming to improve walking movement rehabilitation.
06 Nox Health-DTx: A DTx solution for chronic sleep disorders. It changes the quality of life of patients by improving sleep quality. Their digital therapeutics software combines data, science, AI, and empathy, and it is the first FDA-approved DTx for the treatment of chronic insomnia.


Disney Releases New Concept Art for 'Fantastic Four: First Steps'
Disney Releases New Concept Art for 'Fantastic Four: First Steps'more

Large - scale Seizure of Illegal E - cigarettes at Saint - Ouen Flea Market in France
Large - scale Seizure of Illegal E - cigarettes at Saint - Ouen Flea Market in Francemore

French Health Insurance Cancels Accreditation of Seven Medical Centers over Fraud
French Health Insurance Cancels Accreditation of Seven Medical Centers over Fraudmore

Laos Reaffirms Commitment to People's Health and Well-being at UN Conference
Laos Reaffirms Commitment to People's Health and Well-being at UN Conferencemore

Botswana Golden Grand Prix 2026 to Be Upgraded as Precursor to World Relays
Botswana Golden Grand Prix 2026 to Be Upgraded as Precursor to World Relaysmore

The Indian Temple in New Jersey: A Glimpse of Indian Culture in the United States
The Indian Temple in New Jersey: A Glimpse of Indian Culture in the United Statesmore

South Korea's North Jeolla Province Becomes Candidate Region for 2036 Olympics Bid
South Korea's North Jeolla Province Becomes Candidate Region for 2036 Olympics Bidmore
Trump Administration's Plan to Cut Gavi Funding Sparks Global Concerns
Trump Administration's Plan to Cut Gavi Funding Sparks Global Concernsmore

