Search for information
Two Milestone Cooperation Agreements Mark Africa's Leap towards Vaccine Self-production at the SeconTwo Milestone Cooperation Agreements Mark Africa's Leap towards Vaccine Self-production at the Second Vaccine and Health Products Manufacturing Forum in Cairo
April 15, 2025, 3:33 pm EDT
Two Milestone Cooperation Agreements Mark Africa's Leap towards Vaccine Self-production at the Second Vaccine and Health Products Manufacturing Forum in Cairo
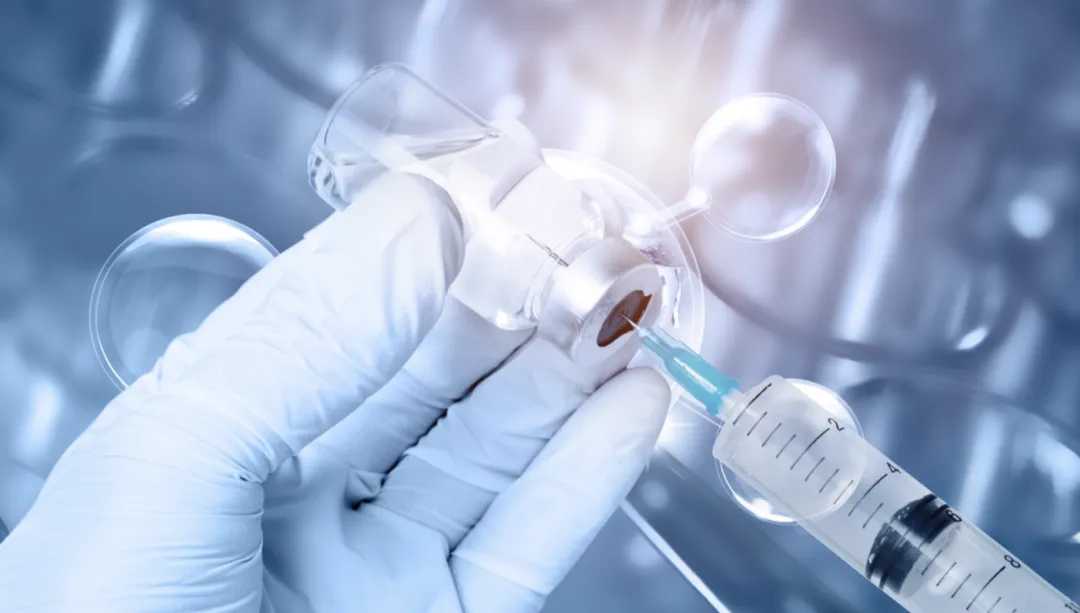
Source: Images from the Internet, if there is any infringement, please contact the removal of

Kit Harington Hints at Lack of 'Eternals' Sequel and MCU Return
Kit Harington Hints at Lack of 'Eternals' Sequel and MCU Returnmore

The Veep show is back on the go! Hundreds of flowers and beauty gods return!-2
The Veep show is back on the go! Hundreds of flowers and beauty gods return!-2more

Unprecedented Layoffs in the US Healthcare System: A Double-Edged Sword
Unprecedented Layoffs in the US Healthcare System: A Double-Edged Swordmore

Chemicals Found in Philips' Silicone Ablation Foam Substitute for CPAP Devices
Chemicals Found in Philips' Silicone Ablation Foam Substitute for CPAP Devicesmore

NVIDIA Plans to Produce $500 - Billion - Worth of AI Equipment in the US
NVIDIA Plans to Produce $500 - Billion - Worth of AI Equipment in the USmore




